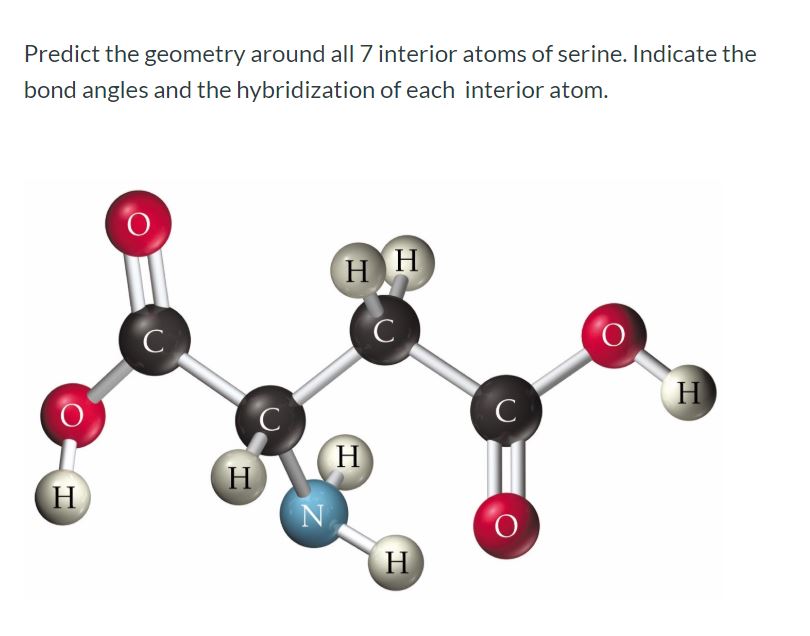
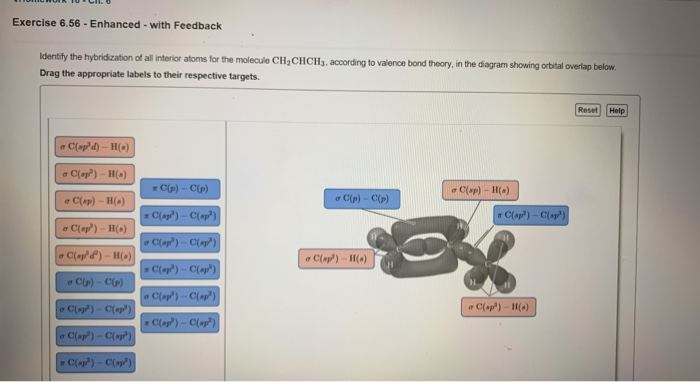
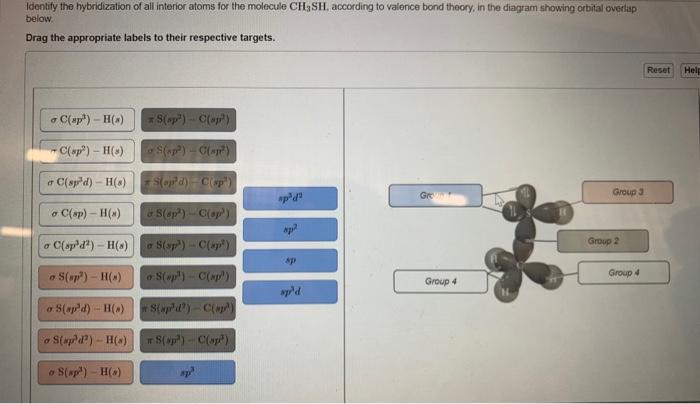
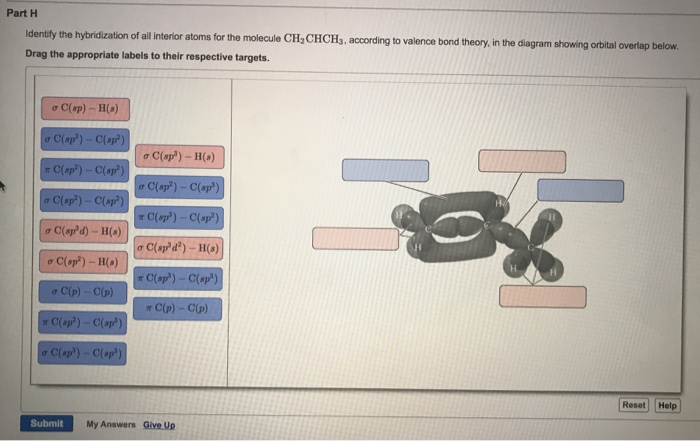
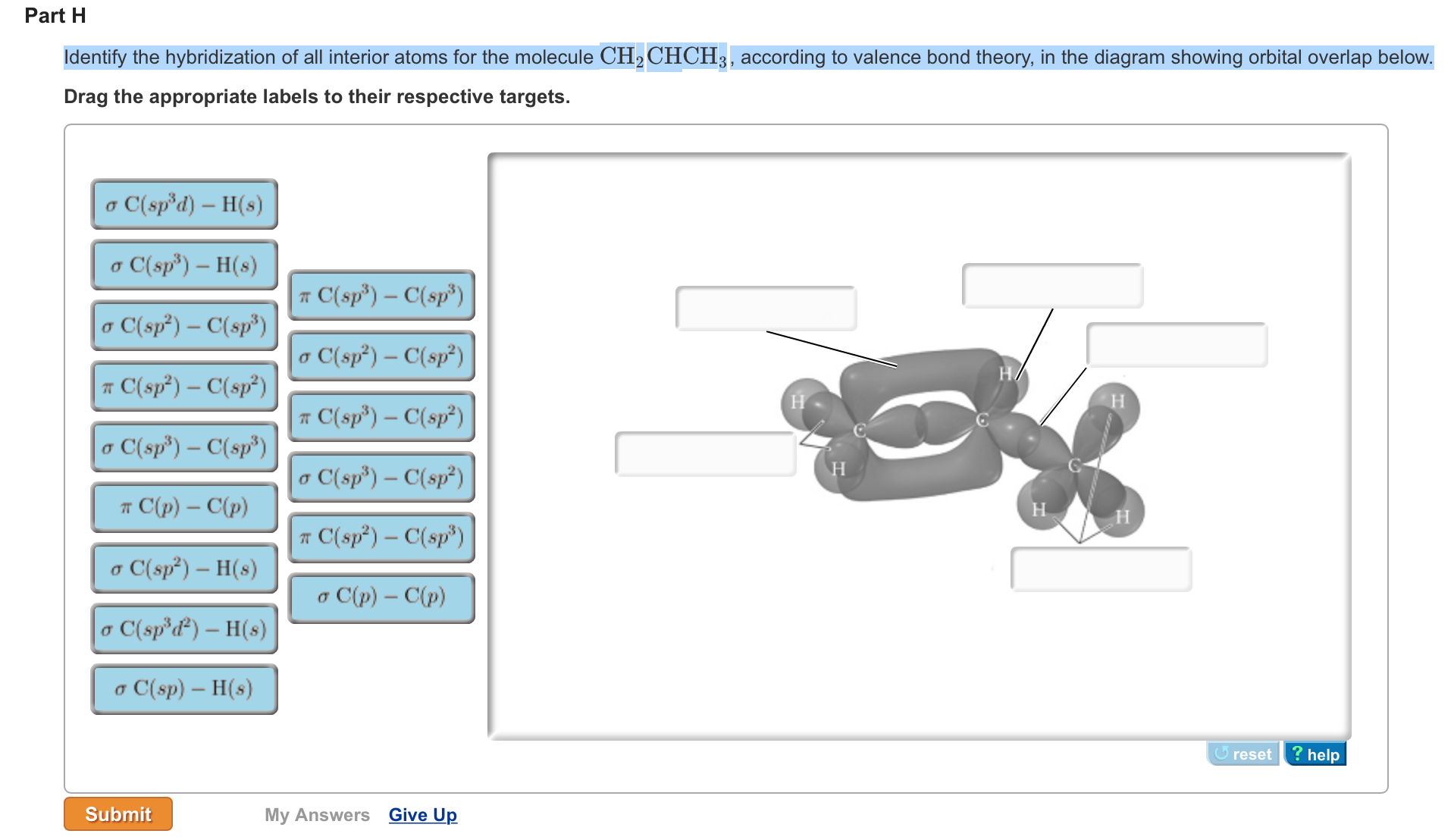
Draw the lewis structure for the molecule ch2chch3. how many sigma and pi bonds does it 30) contain - brainly.com
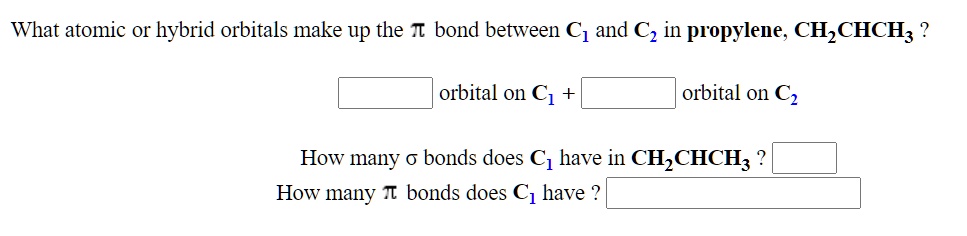
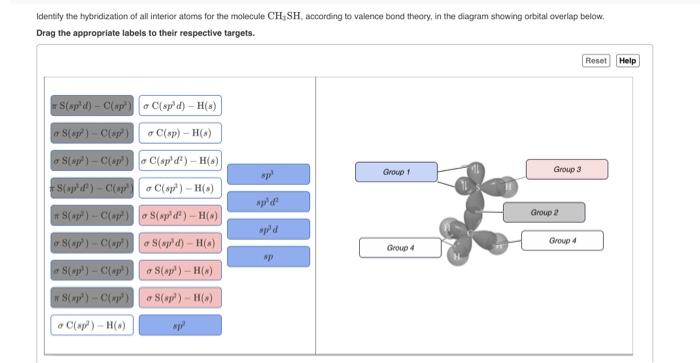
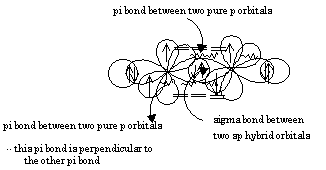
SOLVED: What atomic or hybrid orbitals make up the π bond between C1 and C2 in propylene, CH2CHCH3? π orbital on C1 π orbital on C2 How many bonds does C1 have

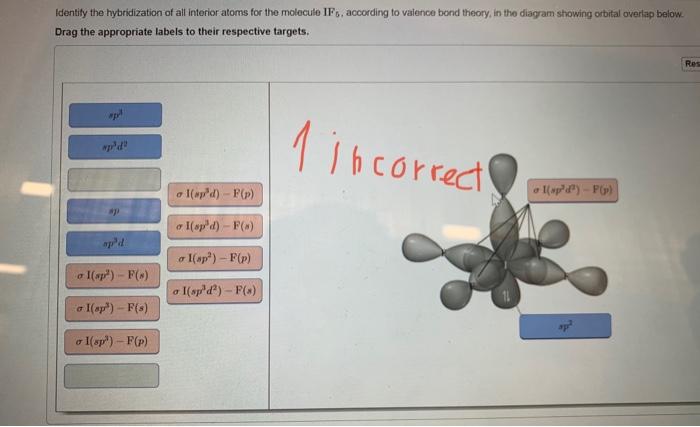
For each atom listed, identify the geometry as one of the following: tetrahedral, square planar, trigonal planar, trigonal pyramidal, or linear. | Homework.Study.com
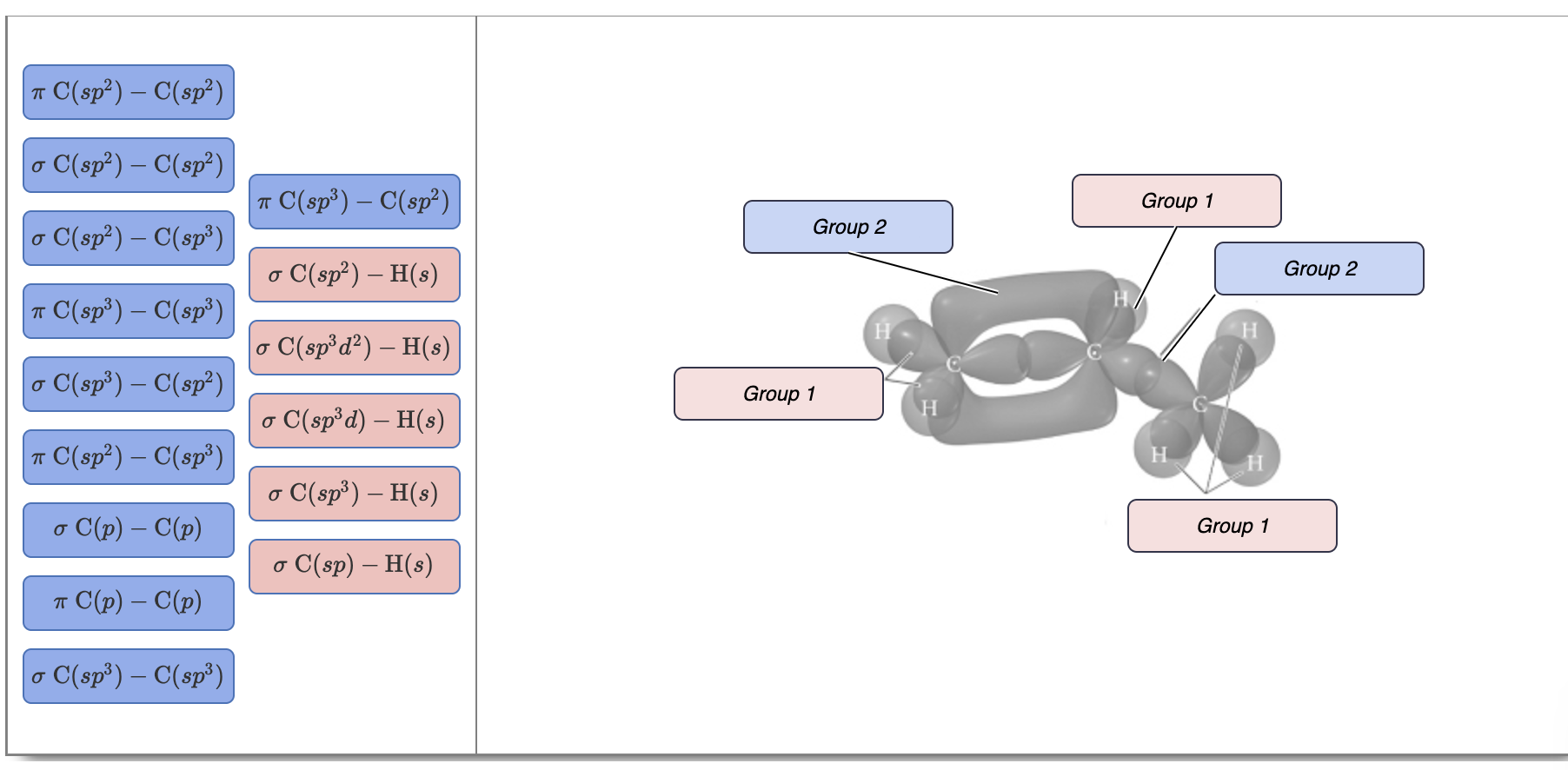
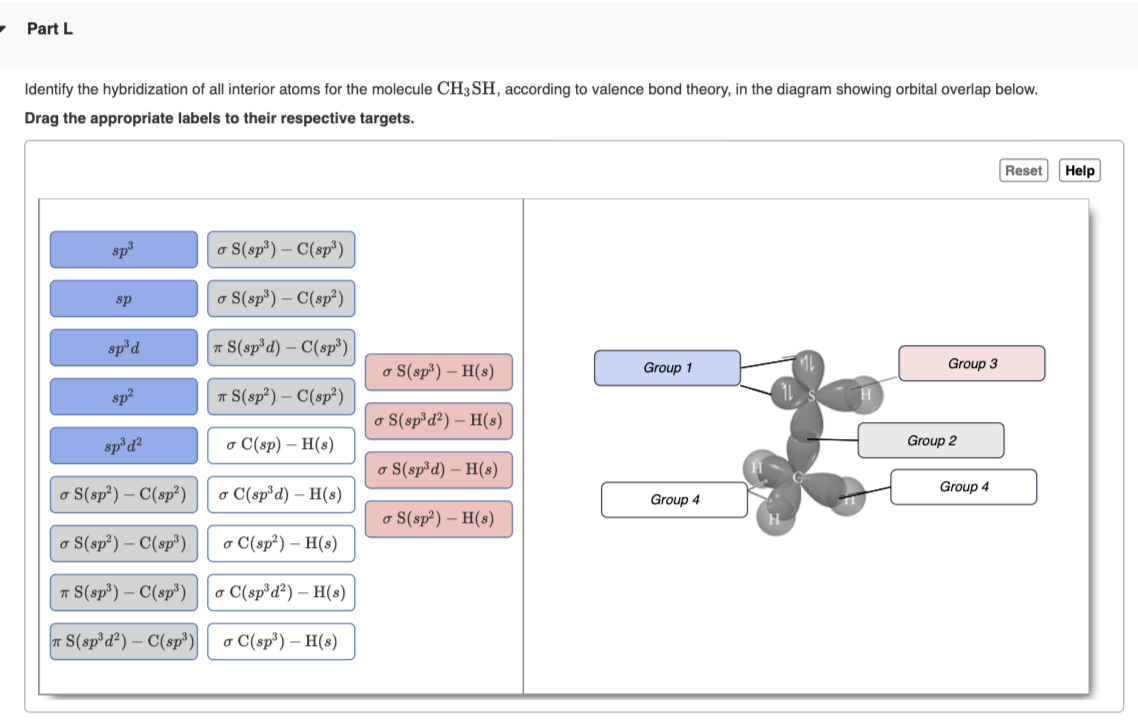
University of Thi-Qar … College Of Science ……….. Department of Chemistry Organic Chemistry Second Stage Lecture 1 ( B
Write the type of hybridisation of each of the carbon atom in the following structures: (i) ch2=c=ch2 (ii) ch3 ch=ch ch3
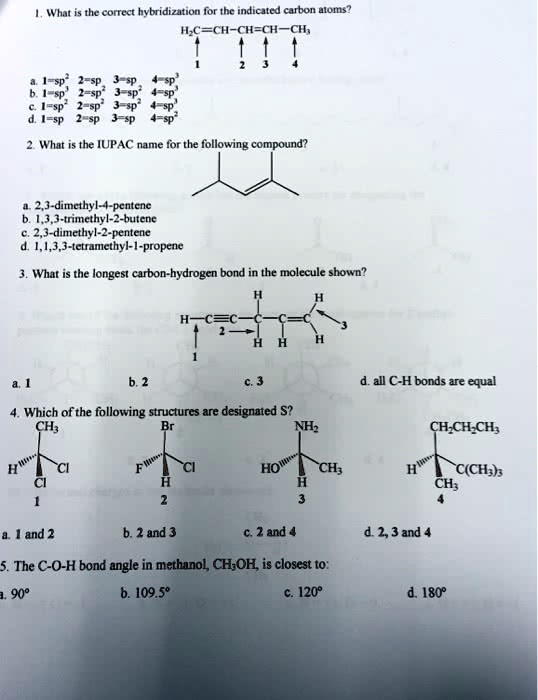
SOLVED: 1. What is the correct hybridization for the indicated carbon atoms? H2C=CH-CH=CH-CH3 a. -sp2, 3-sp3, 4-sp2. 2. What is the IUPAC name for the following compound? a. 2,3-dimethyl-4-pentene b. 1,3,3-trimethyl-2-butene c.

Describe the molecular geometry around each carbon atom in CH2CHCH3 using VSEPR theory. | Homework.Study.com
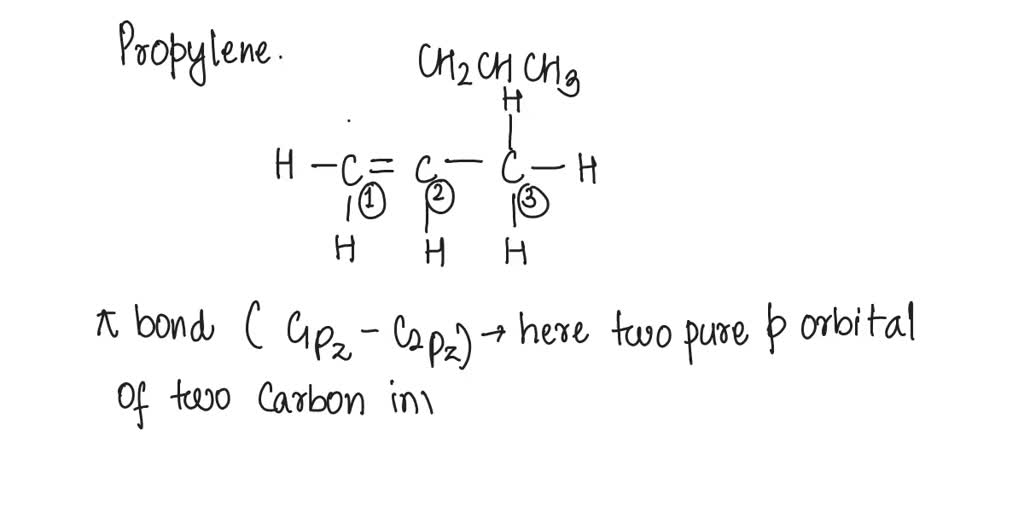
SOLVED: What atomic or hybrid orbitals make up the π bond between C1 and C2 in propylene, CH3(CHCH3)? (C1 is the first carbon in the formula as written: orbital on C1 orbital

Draw the lewis structure for the molecule ch2chch3. how many sigma and pi bonds does it 30) contain - brainly.com