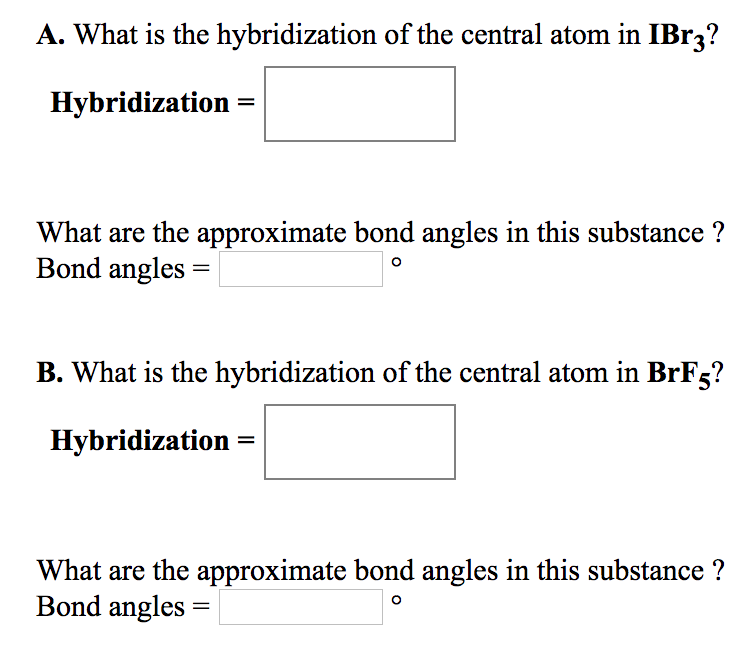
12. Which of the following compounds exhibit d 2sp 3 hybridization? (Select all that apply.) BrF5 ClF5 KrCl4 XeCl2 PCl5 13. Draw the Lewis structure for IF3 and answer the following question.

12. Which of the following compounds exhibit d 2sp 3 hybridization? (Select all that apply.) BrF5 ClF5 KrCl4 XeCl2 PCl5 13. Draw the Lewis structure for IF3 and answer the following question.


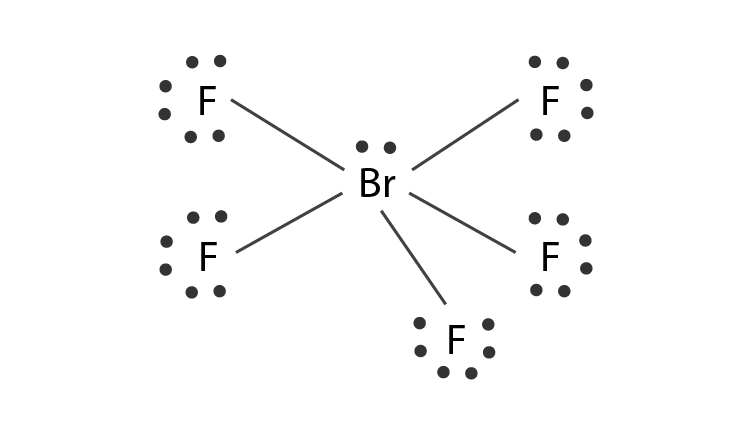


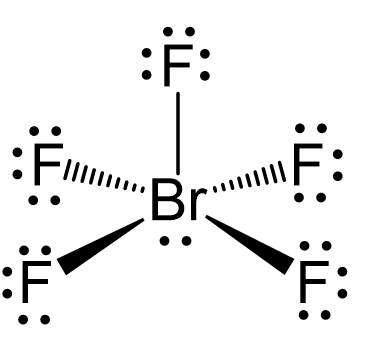






![sp3d2 hybridization is not displayed by : (1) BrF5 (2) SF6 (3) [CrF6]3− (4) PF5 - Brainly.in sp3d2 hybridization is not displayed by : (1) BrF5 (2) SF6 (3) [CrF6]3− (4) PF5 - Brainly.in](https://hi-static.z-dn.net/files/d39/27435abe12f9f9cf4f0688ed61384dea.jpg)